Lamotrigine
From Wikipedia, the free encyclopedia
| This article may require cleanup to meet Wikipedia's quality standards. Please improve this article if you can. (December 2008) |
| This article includes a list of references or external links, but its sources remain unclear because it has insufficient inline citations. Please help to improve this article by introducing more precise citations where appropriate. (December 2008) |
 |
|
|
Lamotrigine
|
|
| Systematic (IUPAC) name | |
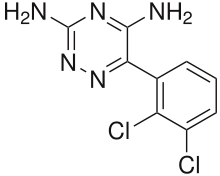
| 6-(2,3-dichlorophenyl)-1,2,4-triazine-3,5-diamine | |
| Identifiers | |
| CAS number | |
| ATC code | N03 |
| PubChem | |
| DrugBank | |
| ChemSpider | |
| Chemical data | |
| Formula | C9H7Cl2N5 |
| Mol. mass | 256.091 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | 98% |
| Protein binding | 55% |
| Metabolism | Hepatic (mostly UGT1A4-mediated) |
| Half life | 24–34 hours (healthy adults) |
| Excretion | Renal |
| Therapeutic considerations | |
| Pregnancy cat. |
C(US) |
| Legal status | |
| Routes | Oral |
Lamotrigine (marketed as Lamictal (IPA: /ləˈmɪktəl/) by GlaxoSmithKline, called Lamictin in South Africa, למוג'ין (Lamogine)[1] in Israel, and 라믹탈 in South Korea and also Lamitor) is an anticonvulsant drug used in the treatment of epilepsy and bipolar disorder. For epilepsy it is used to treat partial seizures, primary and secondary tonic-clonic seizures, and seizures associated with Lennox-Gastaut syndrome. Lamotrigine also acts as a mood stabilizer. It is the first medication since lithium to be granted approval by the U.S. Food and Drug Administration (FDA) for the maintenance treatment of bipolar type I. Chemically unrelated to other anticonvulsants (due to Lamotrigine being a Phenyltriazine), lamotrigine has relatively few side-effects and does not require blood monitoring in monotherapy. The exact way lamotrigine works is unknown. Some think that it is a Na+ (sodium) channel blocker, though it is interesting to note that lamotrigine shares very few side-effects with other, unrelated anticonvulsants known to inhibit sodium channels, (e.g. Oxcarbazepine), which may suggest that lamotrigine has a different mechanism of action. The drug information provided at the time of prescription notes that "it is thought to work by restoring the balance of certain natural substances (neurotransmitters) in the brain." [2] (Lamotrigine is inactivated by hepatic glucuronidation.
However, lamotrigine has also been successful in controlling rapid cycling and mixed bipolar states in persons with more serious psychiatric disorders and who have not received what is considered to be adequate relief from lithium, carbamazepine or valproate, because it is deemed to possibly have significantly more antidepressant potency than either carbamazepine or valproate.[citation needed]
Contents |
[edit] U.S. Food and Drug Administration approval history
| This section does not cite any references or sources. Please help improve this article by adding citations to reliable sources (ideally, using inline citations). Unsourced material may be challenged and removed. (December 2008) |
- December 1994 - for use as adjunctive treatment for partial seizures with or without secondary generalization in adult patients (16 years of age and older).
- August 1998 - for use as adjunctive treatment of Lennox-Gastaut syndrome in pediatric and adult patients, new dosage form: chewable dispersible tablets.
- December 1998 - for use as monotherapy for treatment of partial seizures in adult patients when converting from a single enzyme-inducing anti-epileptic drug (EIAED).
- January 2003 - for use as adjunctive therapy for partial seizures in pediatric patients as young as 2 years of age.
- June 2003 - for the maintenance treatment of adults with Bipolar I Disorder to delay the time to occurrence of mood episodes (depression, mania, hypomania, mixed episodes) in patients treated for acute mood episodes with standard therapy. Additionally, the FDA has noted that findings for Lamictal maintenance treatment were more robust in bipolar depression.
- January 2004 - for use as monotherapy for treatment of partial seizures in adult patients when converting from the anti-epileptic drug valproate (including valproic acid (Depakene); sodium valproate (Epilim) and divalproex sodium (Depakote)).
[edit] Therapeutic uses
[edit] Epilepsy and seizures
Lamotrigine was approved in 1994 by the US FDA for the treatment of partial seizures.[3] Lamotrigine is one of a small number of FDA-approved therapies for seizures associated with Lennox-Gastaut syndrome, a severe form of epilepsy. Typically developing before four years of age, LGS is associated with developmental delays. There is no cure, treatment is often complicated, and complete recovery is rare. Symptoms include the atonic seizure (also known as a "drop attack"), during which brief loss of muscle tone and consciousness cause abrupt falls. Lamotrigine significantly reduces the frequency of LGS seizures, and is one of two medications known to decrease the severity of drop attacks.[4] Combination with valproate is common, but this increases the risk of lamotrigine-induced rash, and necessitates reduced dosing due to the interaction of these drugs.[5]
[edit] Bipolar disorder
Lamotrigine approved in 2003 by the FDA for maintenance treatment of Bipolar I disorder; the first since lithium.[6] While traditional anticonvulsant drugs are predominantley antimanics, lamotrigine is most effective in the treatment and prophylaxis of bipolar depression. Lamotrigine treats bipolar depression without triggering mania, hypomania, mixed states, or rapid-cycling. It has not demonstrated efficacy in the treatment of acute mania.[7] The 2002 American Psychiatric Association guidelines recommended lamotrigine as a first-line treatment for acute depression in bipolar disorder as well as a maintenance therapy.[citation needed]
However, lamotrigine is not indicated on label for the treatment of acute bipolar symptoms. Because the dosage must be slowly increased from a sub-therapeutic level to the therapeutic level, the drug's utility in the management of acute manic symptoms is debatable; typically benzodiazepines or another anticonvulsant will be used to manage the acute mania until the lamotrigine reaches therapeutic blood concentration.[citation needed]
At doses considered sub-therapeutic, lamotrigine is thought to have a mild anti-depressant effect, leading some to question its safety for use in bipolar disorder, as partial remediation of cyclically depressed individuals (especially teens and young adults) has an elevated corelation to suicide until remission attains therapeutically acceptable levels.[citation needed] If lamotrigine's mechanism of action involves an increase in serotonin levels, this may potentially increase the risk of Serotonin syndrome.[citation needed]
[edit] Other uses
Off-label uses include the treatment of peripheral neuropathy, trigeminal neuralgia, cluster headaches, migraines, and reducing neuropathic pain. [8][9][10] Off-label psychiatric usage includes the treatment of depersonalization disorder, bipolar II disorders, schizoaffective disorder, borderline personality disorder, post traumatic stress disorder, and as adjunctive therapy for treatment refractory unipolar depression. [11]
[edit] Mechanism of action
One proposed mechanism of action for lamotrigine involves an effect on sodium channels, although this remains to be established in humans. In vitro pharmacological studies suggest that lamotrigine inhibits voltage-sensitive sodium channels, thereby stabilizing neuronal membranes and consequently modulating presynaptic transmitter release of excitatory amino acids (for example glutamate and aspartate).[12]
[edit] Pharmacokinetics
The pharmacokinetics of lamotrigine are quite complicated, with highly varying half-life and blood plasma levels. Lamotrigine has fewer drug interactions than many anticonvulsant drugs, although pharmacokinetic interactions with Sodium Valproate in particular is an indication for blood monitoring.
[edit] Side effects
Lamotrigine prescribing information has a black box warning about life threatening skin reactions, including Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis.[12] The manufacturer states that nearly all cases appear in the first 2 to 8 weeks of therapy and if medication is suddenly stopped then resumed at the normal dosage. Patients should seek medical attention for any unexpected skin rash as its presence is an indication of a possible serious or even deadly side effect of the drug. Not all rashes that occur while taking lamotrigine progress to Stevens-Johnson Syndrome or Toxic Epidermal Necrolysis It is estimated that 5 to 10 % of patients will develop a rash, but that only one in a thousand patients will develop a serious rash. It is thought that one in 50,000 exposed patients may die from a rash.
Cognitive side effects are common with doses over 50 mg qid (quarter in die - four times daily), as shown in the 2001–2003 Glaxo-sponsored Clinical Trials comparing quality of life between Topiramate and Lamotrigine in healthy volunteers (unpublished).[citation needed] Common side effects include headaches, dizziness and insomnia. Other side effects may include acne and skin irritation, vivid dreams or nightmares, night sweats, body aches and cramps, muscle aches, dry mouth, fatigue, memory and cognitive problems, irritability, weight changes, hair loss, changes in libido, frequent urination, nausea, and other side effects. In very rare cases, Lamotrigine has been known to cause the development of a dangerous rash called Stevens-Johnson syndrome (or SJS). The rash is more common in children, so this medication is often reserved for adults. There is also an increased incidence of this rash in patients who are currently on, or recently discontinued a valproate-type anticonvulsant drug, as these medications interact in such a way that the clearance of both is decreased and the effective dose of lamotrigine is increased.
In clinical trials women were more likely than men to have side effects. This is the opposite of most other anticonvulsants and antipsychotics. It has been suggested that genetic background makes a difference in dosages, with those of non-Caucasian background typically needing lower doses.[citation needed]
Lamotrigine binds to melanin-containing tissues such as the iris of the eye. The long-term consequences of this are unknown.[13]
Use during pregnancy is recommended only if benefits outweigh potential risks. It was also reported on CNN in September 2006 that taking Lamictal during the first trimester of pregnancy can lead to a cleft palate in babies.[citation needed] Lamotrigine is found in breast milk; breastfeeding is not recommended during treatment.
Some patients have reported experiencing a loss of concentration, even with very small doses, while some others have actually reported an increase in alertness and concentration. In general, however, it tends to have less of an impact on concentration relative to other mood stabilizers.[citation needed] GlaxoSmithKline investigated lamotrigine for the treatment of ADHD. The results were inconclusive. No detrimental effects on cognitive function were observed, however, the only statistical improvement in core ADHD symptoms was an improvement on a test, PASAT (Paced Auditory Serial Addition Test), that measures auditory processing speed and calculation ability.[14]
[edit] Availability
GlaxoSmithKline's trademarked brand of Lamotrigine, Lamictal, is manufactured in scored tablets (25 mg, 100 mg, 150 mg and 200 mg) and chewable dispersible tablets (2 mg, 5 mg and 25 mg). Five-week sample kits are also available; these include titration instructions and scored tablets (25 mg for patients taking valproate, 25 mg and 100 mg for patients not taking valproate). Lamotrigine is also available in un-scored tablet form. In 2005, Teva Pharmaceutical Industries Ltd. began selling generic Lamotrigine in the United States, but only in 5 mg and 25 mg chewable dispersible tablets.[15] On 23 July 2008 Teva began offering the full line of generic Lamotrigine in the US.[16] Lamotrigine is also available in generic form[17] in the United States, the United Kingdom and Canada.
[edit] References
- ^ anonymous (2007). "LAMOTRIGINE". www.drug.co.il. http://www.drug.co.il/bygeneric.asp?gen_id=775&drugID=7007&wel=. Retrieved on 2008-04-14.
- ^ DRUG INFORMATION" Sheet, Copyright 2008, First DataBank, Inc. provided by Savon Pharmacy.
- ^ anonymous (19 March 2004). "EFFICACY SUPPLEMENTS APPROVED IN CALENDAR YEAR 2003". FDA/Center for Drug Evaluation and Research. http://www.fda.gov/cder/rdmt/ESCY03AP.HTM. Retrieved on 2008-04-09.
- ^ French, J.A. et al. (2004). Efficacy and tolerability of the new antiepileptic drugs II: Treatment of refractory epilepsy [electronic version]. Neurology. 62:1261–1273.
- ^ Pellock, J.M. (1999). Managing pediatric epilepsy syndromes with new antiepileptic drugs [Special issue, electronic version]. Pediatrics. 104(5): 1106–1116.
- ^ GlaxoSmithKline, 2003
- ^ Goldsmith DR, Wagstaff AJ, Ibbotson T, Perry CM (2003). "Lamotrigine: a review of its use in bipolar disorder". Drugs 63 (19): 2029–50. PMID 12962521.
- ^ Backonja, M. (2004). Neuromodulating drugs for the symptomatic treatment of neuropathic pain. Cur Pain Headache Rep. 8(3):212–6
- ^ Jensen, T.S. (2002). Anticonvulsants in neuropathic pain: rationale and clinical evidence. [Abstract]. Eur J Pain. 6 Suppl A:61–68.
- ^ Pappagallo, M. (2003). Newer antiepileptic drugs: possible uses in the treatment of neuropathic pain and migraine. [Abstract]. Clin. Ther. 25(10):2506–38.
- ^ Barbosa, L. Berk, M. Vorster, M. (2003). A double-blind, randomized, placebo-controlled trial of augmentation with lamotrigine or placebo in patients concomitantly treated with fluoxetine for resistant major depressive episodes. [Abstract]. J Clin Psychiatry. 64(4):403–407.
- ^ a b anonymous (May 2007). "LAMICTAL PRESCRIBING INFORMATION" (PDF). GlaxoSmithKline.. http://us.gsk.com/products/assets/us_lamictal.pdf. Retrieved on 2008-04-09.
- ^ anonymous. "Lamictal, Warnings & Precautions". RxList Inc.. http://www.rxlist.com/cgi/generic/lamotrigine_wcp.htm. Retrieved on 2008-04-09.
- ^ anonymous. "lamotrigine studies". GlaxoSmithKline. http://ctr.gsk.co.uk/Summary/lamotrigine/studylist.asp. Retrieved on 2008-04-09.
- ^ anonymous (17 February 2005). "Press Release, Teva Announces Settlement Of Lamictal Litigation With Glaxosmithkline". Teva Pharmaceutical Industries Ltd.. http://www.tevapharm.com/pr/2005/pr_513.asp. Retrieved on 2008-04-09.
- ^ http://www.tevapharm.com/pr/2008/pr_779.asp
- ^ anonymous (2 March 2005). "Treatment for epilepsy: generic lamotrigine". Department of Health (UK). http://www.dh.gov.uk/en/Healthcare/Medicinespharmacyandindustry/Prescriptions/DH_4104966. Retrieved on 2008-04-09.
[edit] Other sources
- Campbell, G.H. Lutsep, H.L. (2004, 16 December). Trigeminal neuralgia. J. Mendizabal, et al. (Eds). Accessed on March 22, 2005.
- Center for Drug Evaluation and Research. (2005, 1 April). A catalog of FDA approved drug products. Washington, DC: U.S. Food and Drug Administration. Accessed on April 1, 2005.
- Center for Drug Evaluation and Research. (2005, 1 April). Electronic orange book: Approved drug products. Washington, DC: U.S. Food and Drug Administration. Accessed on April 1, 2005.
- Curry, W.J. and Kulling, D.L. (1998). Newer Antiepileptic Drugs: Gabapentin, Lamotrigine, Felbamate, Topiramate and Fosphenytoin. American Family Physician. 57(3): 513-?.
- GlaxoSmithKline. (2003, June 23). Lamictal: First medication since Lithium approved for long-term maintenance treatment of bipolar disorder. Press Release.
- GlaxoSmithKline. (2004, January 14). For epilepsy it is used to treat partial seizures, primary and secondary tonic-clonic seizures, and seizures associated with Lennox-Gastaut syndrome. Lamictal (lamotrigine): Prescribing information. Accessed on February 2, 2005.
- GlaxoSmithKline UK. (2004, February 9). Lamictal combined tablets. electronic Medicines Compendium. Accessed on February 2, 2005.
- Glauser, T.A. Morita, D.A. (2002, January 30). Lennox-gastaut syndrome. (eds). Accessed on March 22, 2005.
- Huntington’s Outreach Project for Education. (2004, December 8). Lamotrigine disease mechanism V: Glutamate toxicity Stanford University. Accessed on March 12, 2005.
- Ochoa, J.G. & Riche W. (2005, 2 March). Antiepileptic drugs: An overview. E.A. Passaro, et al. (Eds). Accessed on March 12, 2005.
[edit] External links
- FAQ: Psychiatric Uses of Lamotrigine (Lamictal), by Ivan K. Goldberg, MD. Includes many references from the medical literature.
- Center for Drug Evaluation and Research: Lamictal - documents related to the FDA approval process, including medical reviews and correspondence letters.
- Epilepsy South Africa: MEDICATION FOR EPILEPSY - an Epilepsy FAQ with a list of medicines for treatment thereof, includes Lamotrigine with South African trade name Lamictin.
- Adverse Reactions - Reported adverse reactions and side effects.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||


